Abstract

Introduction: Survival of patients with high-risk large B-cell lymphoma (LBCL), particularly those with biological risk factors, including chromosomal translocation of BCL2 and MYC oncogenes (double hit; DH) or deletion of 17p/TP53 is suboptimal in response to standard rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) immunochemotherapy. We designed a Nordic Lymphoma Group (NLG-LBC-06) phase II trial to evaluate feasibility and efficacy of a biological risk-adapted treatment strategy in young patients with high-risk aggressive B-cell lymphoma.
Methods: Patients aged <65 years with high-risk aggressive B-cell lymphoma (age adjusted International Prognostic Index (aaIPI)≥2 and/or site-specific risk factors for central nervous system (CNS) relapse) were included. All patients received two cycles of R-CHOP-21 with high dose methotrexate (HD) on day 15 and depending on the biological risk factors either four additional courses of biweekly R-CHOP with etoposide (R-CHOEP-14; no biological risk factors) or four courses of dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab (DA-EPOCH-R; biological risk factors). In addition, one course of R-HD-cytarabine was given to all patients. Biological high-risk was defined as a presence of at least one of the following factors: C-MYC translocation, DH, 17p/TP53 deletion, co-expression of MYC and BCL2 (double protein expression; DPE), P53+ and/or CD5+. Central pathology review was performed by national referral pathologists. Primary end point was 3-year failure free survival (FFS) of the patients with biological risk factors.
Results: Between Aug 2017 and Jan 2021, 127 patients were recruited across 14 Nordic centers. At central pathology review, four cases were excluded due to unsuccessful stratification. Median age of the eligible patients was 55 years (range 19-64). Majority of the patients had DLBCL not otherwise specified (n=102; 83%), stage IV disease (n=84; 68%), elevated LDH (n=107; 87%), or B-symptoms (n=72; 59%). C-MYC translocation, DH, 17p/p53 deletion, DPE, P53+ and CD5+ was observed in 20 (17%), 14 (11%), 19 (17%), 39 (32%), 17 (14%) and 8 (7%) of the cases, respectively. Sixty-one patients (50%) were stratified to the biological high-risk group. Most patients (n=112; 91%) received full treatment schedule. In addition, twenty-three patients (19%) received radiotherapy per protocol. Of the 111 patients who underwent response evaluation at the end of immunochemotherapy, 87 (78%) achieved a complete metabolic remission.
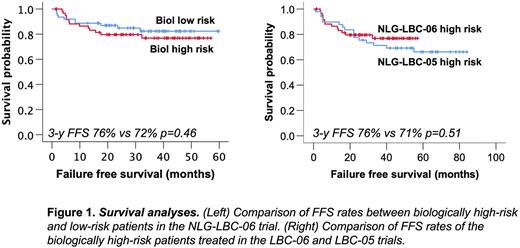
Treatment failure was recorded in 23 (19%) patients; seven due to adverse event, five due to primary refractory disease and 10 due to lymphoma relapse or progression during follow-up, including two CNS events and one death from other disease. After a median follow up of 37 months (1-63), 3-year FFS, progression free survival (PFS) and overall survival (OS) rates were 79%, 83% and 90%, respectively. Outcome was comparable in biologically high- and low-risk patients (Figure 1, left). Based on a preplanned historical comparison, and after correction for age and aaIPI, FFS of the biological high-risk group (HR, 0,89; 95% CI 0,38-1,71; p=0,58; Figure 1, right) and the entire NLG-LBC-06 study population (HR, 1,03; 95% CI 0,60-1,79; p=0,91) were comparable to FFS of the patients treated in our previous NLG-LBC-05 trial (Leppä et al., 2019). In a multivariate analysis with age and aaIPI, 17p/TP53 deletion remained the only significant biological risk factor for progression and death.
Conclusions: Stratification according to biological risk factors is feasible. Safety and efficacy results are encouraging and comparable to our previous study with low number of treatment failures and favorable survival rates. Furthermore, our results indicate that treatment intensification can overcome the adverse impact of biological risk factors, apart from 17p/TP53 deletion.
Disclosures
Leppa:Roche: Consultancy, Research Funding; Gilead Sciences: Consultancy, Honoraria; BMS: Consultancy, Research Funding; Bayer: Research Funding; Orion Pharma: Consultancy; Pfizer: Consultancy; Beigene: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Genmab: Research Funding; Nordic Nanovector: Research Funding. Jørgensen:Gilead: Consultancy; Novartis: Consultancy; Orion Pharma: Consultancy; BMS: Consultancy; Incyte: Consultancy; Roche: Consultancy. Drott:Respiratorius AB: Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Honoraria; Roche: Honoraria. Mannisto:Novartis: Consultancy, Honoraria. Larsen:Genentech: Research Funding; Roche: Consultancy; Novartis: Consultancy; Gilead Sciences: Consultancy; Bristol Myers Squibb: Consultancy. Sunela:Roche: Consultancy, Other: Studies & fees for presentations; Gilead: Consultancy; Bayer: Consultancy, Other: Studies; Exelis: Other: Studies; Incyte: Consultancy, Other: Studies; Eisai: Other: Studies; MSD: Consultancy, Other: Studies; HutchMed: Other: Studies; Boehringer Ingelheim: Other: Studies; Ispen: Consultancy, Other: Fees for Presentations; Janssen-Cilag: Consultancy, Other: Fees for Presentation; Novartis: Consultancy; Merck-Pfizer: Consultancy, Other: Presentation fees; KyowaKirin: Consultancy, Other: Presentation fees; Takeda: Other: Presentation fees; Astellas: Other: Presentation fees; Amgen: Other: Presentation fees. Brown:Roche: Consultancy; Novartis: Consultancy; Gilead: Consultancy. Holte:Nordic Nanovector: Honoraria, Other: Safety committee; Incyte: Honoraria, Other: Advisory board; Genmab: Honoraria, Other: Safety committee; Gilead: Honoraria, Other: Advisory board; Novartis: Honoraria, Other: Advisory board; Takeda: Honoraria, Other: Advisory board.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal